Preclinical Studies: Xenograft (efficacy) and Pharm/Tox (safety)
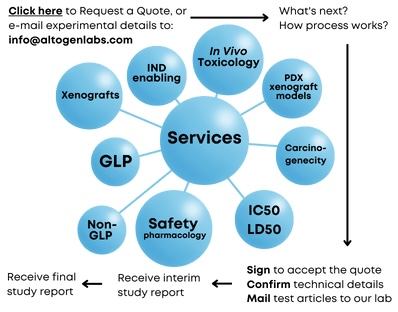
Our services include safety in vivo toxicology studies (for IND applications), efficacy studies (for regulatory submissions), drug discovery (patent applications), drug development, and other types of R&D studies including safety toxicology, biodistribution, ADME, and imaging studies. Preclinical research studies often require a sequence of experiments utilizing various scientific methods to demonstrate test article efficacy, safety, and mechanism of action. Altogen scientists have years of experience and expertise that includes all types of nonclinical contract research studies, cell and molecular biology R&D services, pharmacology, and toxicology safety studies that are required for IND.
Altogen Labs is a GLP compliant contract research laboratory that provides pharma/biotechnology research services for pharmaceutical, biotechnology, and academic institutions worldwide. We provide a variety of biology CRO in vivo services, including 92 in-house validated CDX models, 34 PDX xenograft models, and 12 syngeneic murine models. Our in vitro services include IC50 assays, 28-day development of stable cell lines, gene function and gene expression analysis by qPCR (mRNA) and automated Western Blot system Wes (protein expression), RNAi and CRISPR lab services, transient and stable transfections, test compound liposome encapsulation for tissue-targeted delivery, and more.
Technical support and QA/QC teams ensure and support the company’s high quality products and services. We support clients from study design phase through patent or regulatory submissions (100% of IP belongs to the client). Altogen Labs offers instant quotes, comprehensive service packages, and competitive prices. We are happy to discuss your experimental details to help generate accurate price quotes and timeline estimates (please e-mail study details to info@altogenlabs.com, or call us at 512-433-6177).